As is well known, cosmetic products require a thorough toxicological evaluation to verify that they are safe for human use, and to ensure their compliance with EU Cosmetic Regulation 1223/2009, to enable them to be marketed in the European Union.
A cosmetic product consists of various cosmetic ingredients. Sometimes there are few, other times there are many within its composition. Therefore, a cosmetic product cannot be declared safe and compliant if there is no information about the cosmetic raw materials it contains.
For this reason, cosmetic raw materials that are intended to be marketed in the European Union must meet certain requirements, including the ones mentioned in the EU Cosmetic Regulation 1223/2009 in order to guarantee that their use is safe.
Common challenges in marketing cosmetic raw materials in the European Union
Manufacturers and suppliers of raw materials for cosmetics often encounter obstacles when attempting to market in the European Union due to insufficient information regarding at least one of the following aspects:
- Toxicological information on the cosmetic ingredients contained in the raw materials and on raw materials as a product.
- Information on safety during the use of raw materials (such as Safety Data Sheets).
- Information about the quality of raw materials.
- Other relevant information such as the complete composition, physical-chemical characteristics, instructions for use, efficacy, compliance with different regulations, etc.
That is why conducting a comprehensive toxicological evaluation on raw materials is crucial for companies aiming to sell their products to European manufacturers. With this evaluation in hand, raw material suppliers can enhance sales opportunities, making their ingredients viable for use in a diverse range of cosmetic products throughout the European Union.
This step is essential for engaging with major players like L’Oréal, Estée Lauder, and others, as it demonstrates a commitment to quality and ensures the utmost effectiveness and safety for end-customers. This non-negotiable approach to quality establishes these companies as serious partners in the industry.
What is a Toxicological Evaluation of a Raw Material?
Raw materials, which are composed of one or more cosmetic ingredients, essentially consist of chemical substances.
A toxicological evaluation involves the calculation, compilation, and research of toxicological information about a specific chemical substance, which is crucial when such information has not been previously established or is not readily available in public sources. This critical evaluation must be conducted by a qualified safety assessor.
To perform this evaluation, the safety assessors at Taobé Consulting refer to the Scientific Committee on Consumer Safety (SCCS) Notes of Guidance for Testing and their Safety Evaluation (12th Revision). These notes outline the basic toxicity testing procedures necessary for assessing various human health-related toxicological endpoints. These procedures are internationally recognized, resulting from long-standing scientific consensus.
According to the SCCS’s Notes of Guidance, a typical toxicological and safety evaluation comprises the following elements:
- Hazard identification: Identifies the intrinsic toxicological properties of the substance, i.e. whether it has the potential to damage human health.
- Exposure assessment: Calculated based on the declared functions and uses of a substance as a cosmetic ingredient, the amount present in the respective cosmetic product categories and their frequency of use.
- Dose-response assessment: For the relationship between the exposure and the toxic response, a Point of Departure (PoD) is determined. A No Observed Adverse Effect Level (NOAEL) has been used as PoD.
- Risk characterisation: Focuses on systemic effects. A Margin of Safety (MoS) is calculated.
What are the documents needed to perform this toxicological evaluation?
Each raw material is unique, with a specific composition and characteristics. This is why a case-by-case analysis will be carried out to determine what information is necessary for the toxicological evaluation of each raw material.
The easiest way to proceed is to collect all available documents and tests and, depending on the nature of the cosmetic ingredients and the data found in the documents, we advise on the tests and other actions to be performed.
In general, the information required includes the following:
- Complete composition of the raw material, detailing INCI names and including impurities. In the event that a cosmetic ingredient does not have an INCI name, Taobé can also help with the INCI registration process.
- Chemical and physical specifications of the raw material.
- Chemical and physical specifications of cosmetic ingredients (chemical identity, physical form, solubility, etc.). For ingredients classified as nanomaterials, we will need particle size information.
- Type of cosmetic ingredient (emollient, surfactant, viscosity controlling, etc.).
- Cosmetic product categories in which the raw material may be used.
- Maximal concentration in finished cosmetic product.
- Manufacturing process.
- Marketing material on the virtues of the raw material.
- Maximal levels they intend to market the raw material (1, 10, 100 tonnes per year).
- All tests available regarding acute toxicity, irritation and corrosivity, skin sensitisation, mutagenicity/ genotoxicity, repeated dose toxicity, phototoxicity, reproductive toxicity, developmental toxicity, carcinogenicity, etc.
- Clinical data.
- Epidemiological studies.
- Information derived from accidents.
- Data from Post-Marketing Surveillance (PMS) or other human data.

A very important thing to keep in mind is that animal experiments are prohibited in the European Union for cosmetic purposes:
- The ban of animal testing on finished products has been in force since September 2004.
- The ban of animal testing on a cosmetic ingredient or mixture of cosmetic ingredients has been in force since March 2009, with the exception of repeated-dose toxicity, reproductive toxicity, and toxicokinetics tests, for which the ban came into force in March 2013.
Based on the above, we can only take into account animal studies that have been carried out before the ban and/or validated non-animal methods (NAMs).
What are the benefits of having a toxicological evaluation available?
At the end of the process, Taobé will be able to develop a Dossier including:
- A full toxicological assessment report of the raw material, according to the SCCS Notes of Guidance, demonstrating the safety of the cosmetic ingredient/s according to the utmost authority in Europe. This report can be provided to clients and Responsible Persons to do the corresponding notifications and have peace of mind that the raw material has been thoroughly assessed and will hold up to scrutiny even by regulators.
- A CLP SDS, to provide to importers for REACH notification.
- A Raw Material Regulatory Identity Card that Taobé will create in English and Japanese (if needed), based on the toxicological assessment. It will be a recap of all the key information European manufacturers will be looking at, such as:
- All regulatory information on the raw material.
- Maximal concentration in a finished cosmetic product category (hand cream face cream, body cream, nail polish, etc.) to remain safe and EU compliant.

Steps and lead time for a toxicological evaluation
The time the toxicological evaluation takes will depend on:
- The type of raw material (amount of ingredients within its composition and its nature)
- The information and tests already available.
- The information and tests that are missing and that need to be performed.
- The time it will take to perform the missing tests.
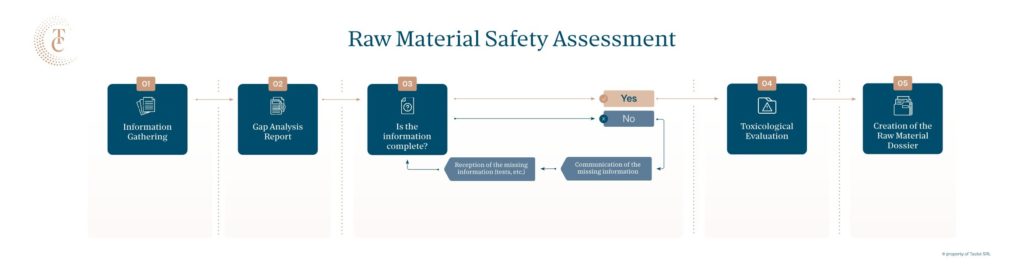
As mentioned before, the simplest way to go is to collect all documents and tests available (the information must be in English) and perform a Gap Analysis to outline the missing information. This will take around 2 weeks.
This Gap Analysis will include a list of missing information and tests needed to be performed in order to complete the toxicological evaluation.
Once the information is received, we can complete the toxicological assessment report and develop the dossier, which will take around 2 weeks.
If you need additional information on our toxicological evaluation services, please visit our page on cosmetics evaluation of raw materials. Alternatively, feel free to reach out to us via our contact page. We’re here to assist you every step of the way.
Flow chart of the toxicological report process: