What Is The Challenge Test in cosmetics?
The challenge test in cosmetics evaluates the effectiveness of a cosmetic product’s preservative system during production, storage, and the entire period of use by the end consumer. It is conducted during the product’s development phase. Essentially, the test simulates the microbiological stress the product will encounter under normal conditions of use and storage throughout its commercial life.
Regulation (EC) No. 1223/2009, Annex I, point 3, addresses the microbiological quality of cosmetic products. It specifies that the results of the Challenge Test must be included in the safety report. Similarly, the SCCS guidelines state that all cosmetic products susceptible to deterioration under normal conditions of use and storage must undergo challenge tests to ensure their microbiological stability.
Principles Upon Which the Challenge Test Is Based
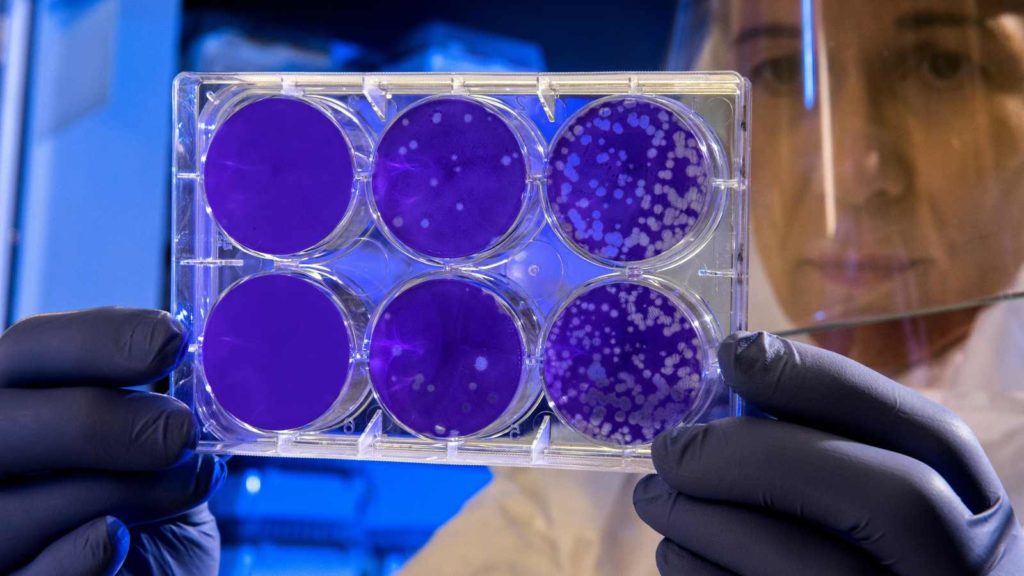
The challenge test involves artificially contaminating the finished product by inoculating it with microorganisms. This is followed by an evaluation of how effectively the contamination is reduced. The contamination levels must comply with the microbiological limits for categories 1 and 2 as defined by the European standard UNI EN ISO 17516:2014 and referenced in the SCCS guidelines, which are summarised in the table below:
There is no universally established method for performing the challenge test. Instead, guidelines provide methods, leaving the Responsible Person to decide the specific details of the test to be used.
The most commonly referenced method is the UNI EN ISO 11930:2019 standard, which defines various parameters, including analytical methods, the strains of microorganisms used, and the interpretation of test results.
According to the UNI EN ISO 11930:2019 standard, the microorganisms used in the challenge test are:
- Pseudomonas aeruginosa
- Staphylococcus aureus
- E. coli
- Candida albicans
- Aspergillus brasiliensis
Phases of the Challenge Test
The challenge test basically consists of the following phases:
- A preliminary evaluation of the sample is essential to ensure it is not already contaminated before the test begins.
- Preparation of the inoculum: it consists of a series of steps in which the strains are transplanted into the most suitable culture medium in order to allow them to grow, these cultures are then washed and centrifuged until the desired suspension is obtained. The required range for inoculation is usually between 10 6 and 10 8 CFU/ml;
- Control of the survival of microorganisms at various time intervals: T0, 24, 48 hours, 7, 14, 21 and 28 days after inoculation.
The experimental execution of microbial controls and challenge tests must be performed, supervised, and validated by a microbiologist. As stated earlier, the Responsible Person is required to ensure the effectiveness of product storage through verification tests.
Consequently, the choice of methodology rests with the Responsible Person and plays a crucial role in safeguarding the health of the cosmetic’s end user. To understand fully the role and responsibilities of the Responsible Person, please check our article on the topic.
Interpretation of Challenge Test Results
Below are the data that can be extrapolated from the table that shows the acceptability criteria, CRITERION A and CRITERION B according to the UNI EN ISO 11930:2019 standard:
Criteria A:
- For bacteria, the product must demonstrate no less than a 3 log reduction from the initial count at 7 days, and no increase from the 7 day count at days 14 and 28.
- C. albicans must be reduced by no less than 1 log at 7 days and show no increase from the 7 day count at days 14 and 28.
- A. brasiliensis must show no increase from the initial count at 14 days and no less than a 1 log reduction at 28 days.
Criteria B:
- For bacteria, the product must demonstrate no less than a 3 log reduction from the initial count at 14 days and no increase from the 14 day count at day 28.
- C. albicans must be reduced by no less than 1 log at day 14 and show no increase from the 14 day count at day 28.
- A. brasiliensis must show no increase from the initial count at 14 and 28 days.
In conclusion, in accordance with Criterion A:
- the microbiological risk is acceptable: the cosmetic product is considered protected against microbial proliferation and it is not necessary to consider other factors that are independent of the formulation.
- Compliance with Criterion B: Microbiological risk is acceptable, but the microbiological risk assessment should also take into account control factors independent of the formulation, such as packaging characteristics to reduce microbiological risk.
- Non-compliance with either of the two criteria indicates that the preservative system is not suitable for preserving the cosmetic product analysed.
Products That Do Not Require the Challenge Test

As specified in the UNI EN ISO 29621:2017 standard, certain products are considered to have a low risk of microbiological contamination, making the challenge test unnecessary in these cases.
This standard assists cosmetics manufacturers and regulatory bodies in identifying finished products that, based on a risk assessment, pose a low risk of microbial contamination during production and/or intended use. Such products are exempt from the application of international microbiological standards for cosmetics.
According to this standard, the criteria to be taken into account are:
- Water activity (Aw) is an index that reflects the amount of water in a product. Products with low or no water content are considered to have a low microbiological risk. A product is classified as low risk when its Aw value is less than 0.6.
- pH values: when the pH is very acidic (< 3.0) or very basic (> 11) the product is considered low risk;
- Alcohol content: products with an alcohol content of more than 20% are considered low risk;
- Raw materials hostile to microbial proliferation;
- Conditions under which production is carried out;
- Type of packaging: for example, single-use products or spray products are considered low risk.
” In-use test “
For cosmetic products without a preservative system, where the challenge test is not applicable, the microbiological risk and safety can be assessed through the in-use test. This in vivo test involves distributing the product to a variable number of consumers, who use it according to its intended purpose.
After a usage period of 4 to 12 weeks, the used products are evaluated microbiologically to determine whether the microbial values remain within the acceptable limits established for finished products.
Microbiological Analyses
Except for products classified as having low microbiological risk—such as those with extreme pH levels, low or no water content, alcohol-based formulations, or raw materials that inhibit microbial growth—all finished cosmetics must undergo microbiological testing for each batch.
In addition to being subject to the challenge test, these further tests ensure compliance with the microbiological specifications defined for categories 1 and 2 by the European standard UNI EN ISO 17516:2014, as summarised in the previously referenced table.
Annex I of Regulation (EC) No 1223/2009 specifies that microbiological specifications must be included in the safety assessment for each cosmetic product. Particular attention should be given to products intended for use on sensitive areas of the body, such as around the eyes or mucous membranes, and to those designed for specific populations, such as children under three years of age.
Microbiological analyses, required for each production batch, are essential to ensure the product is free from contamination and to safeguard the safety and health of the end user.
However, the execution of microbiological tests, including the challenge test, does not apply to products classified as having a low risk of microbiological contamination, as defined by the UNI EN ISO 29621:2017 standard. As previously discussed, these products include:
- Water activity (Aw) is an index that reflects the amount of water in a product. Products with low or no water content are considered to have a low microbiological risk. A product is classified as low risk when its Aw value is less than 0.6.
- pH values: when the pH is very acidic (< 3.0) or very basic (> 11) the product is considered low risk;
- Alcohol content: products with an alcohol content of more than 20% are considered low risk;
- Raw materials hostile to microbial proliferation;
- Conditions under which production is carried out;
- Type of packaging: e.g. single-use products or spray products are considered low risk
Do you have concerns about your product’s compliance? Unsure whether the necessary tests have been conducted?
If you’re looking for a reliable partner to handle this process for you, contact us—we’ll help ensure your product achieves 100% compliance.



