Manufacturing cosmetic products involves numerous quality decisions and responsibilities, including raw material selection, formula compliance, meeting clients’ expectations, adhering to market requirements, and data collection.
What if you had a regulatory partner to relieve you from some of this burden, making your life and the lives of the cosmetic brands you work with much easier?
We can:
At Taobé, we work directly with cosmetic raw material suppliers on INCI registration, (eco-) toxicological profiles, safety assessments, claims efficacy, and more. This allows us to build a comprehensive database of ingredients with specific characteristics (vegan, natural, innovative, etc.), all fully documented according to Cosmetics Regulation requirements.
Share with us the specifications of the cosmetic raw materials you are looking for (e.g., SPF, natural, vegan, Japan), and we will prepare a shortlist of ingredients from our database that match your criteria. If we do not have exactly what you need, we will connect you directly with the raw material suppliers we collaborate with.
We have worked successfully with many cosmetic manufacturers who consistently provide high-quality and thorough documentation in a timely manner. These manufacturers are listed in our “best choice” database, which we promote among our international clientele.
We can also direct clients who seek a manufacturer or private labeller to your sales department, depending on the product category and the claims they wish to label.
We can also direct clients who seek a manufacturer or private labeler to your sales department, depending on the product category and the claims they wish to label.
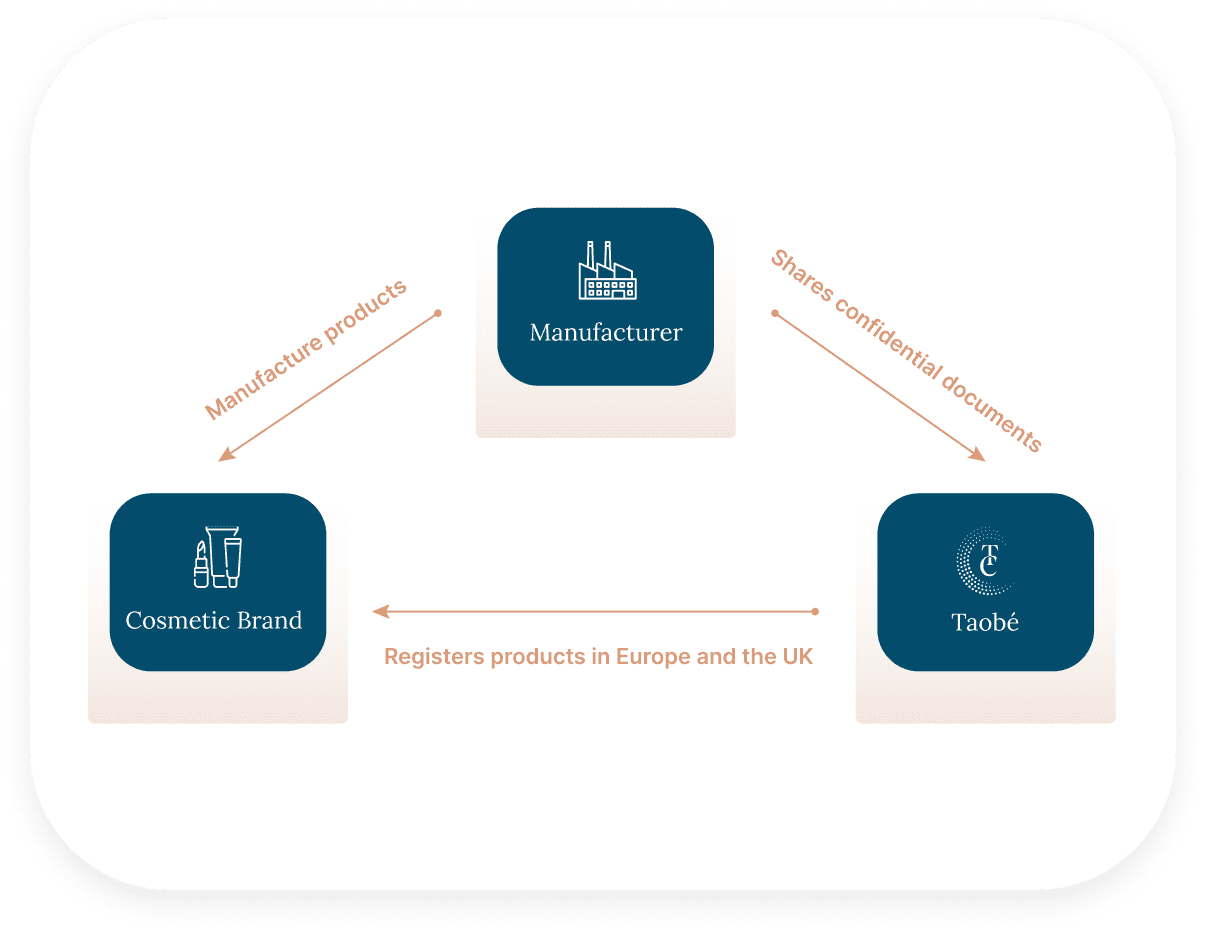
To register a product in Europe and the UK, a product information File needs to be created thanks to all documents and information related to the products and its ingredients. That means that everything confidential about a product (formula, raw material trade names and suppliers), will need to be disclosed.
This means that for a cosmetic brand to be able to expand in Europe, the contract manufacturer would have to disclose its most valuable asset : its know-how.
By partnering with Taobé Consulting, you ensure a firewall between you and your client. No confidential data will ever be shared with your client cosmetic brand as an NDA will be signed and Taobé will take care of all the registrations steps while keeping every data confidential. In the end, all parties get what they want : the brand is able to expand in Europe while the manufacturer keeps everything confidential.
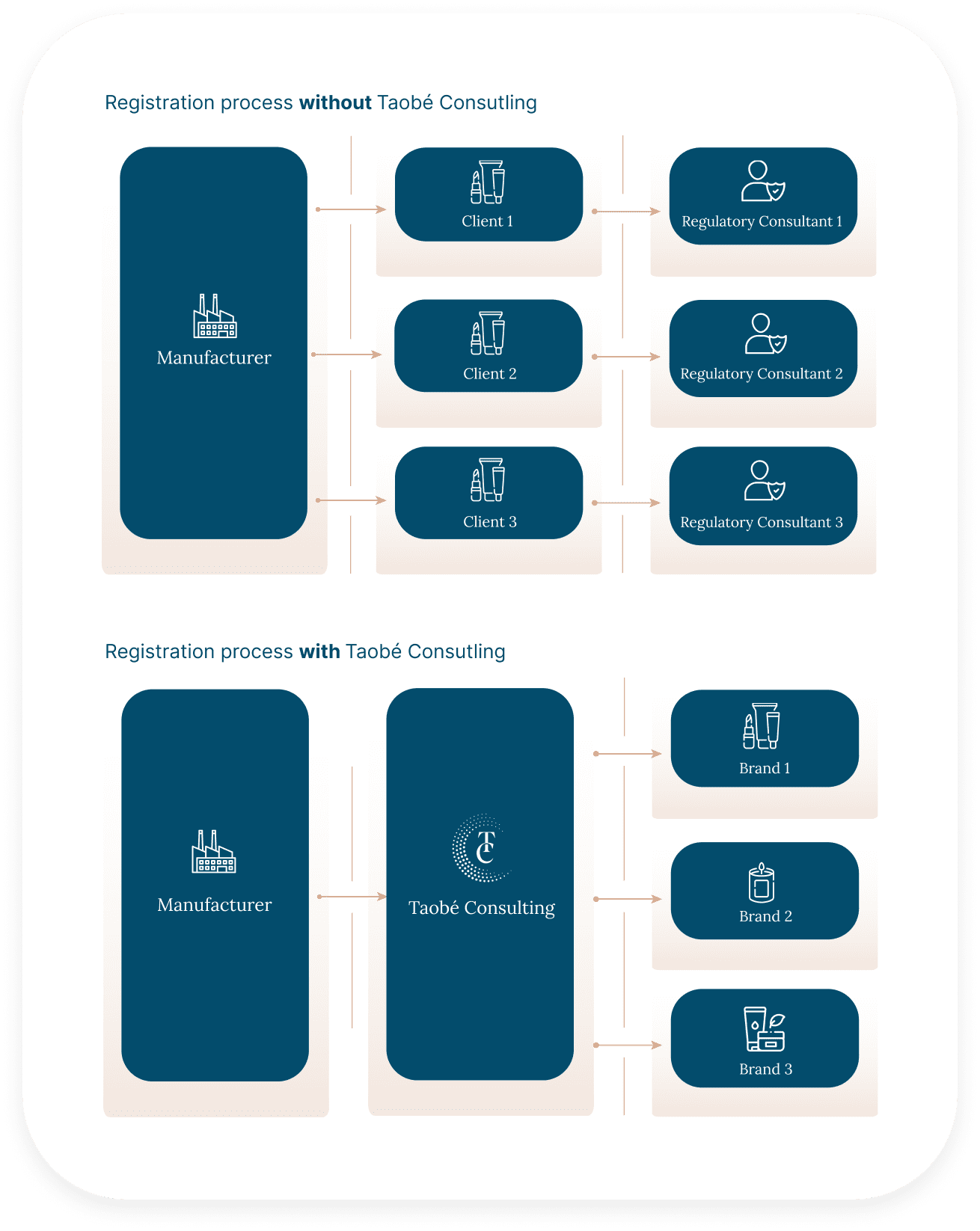
If you ever worked with several client cosmetic brands willing to expand in Europe, you know how time-consuming it is to share documents (often the same) to multiple people, several times.
By partnering with Taobé Consulting, you make sure documents are only shared once and to only one point of contact, through a document management system designed to keep the document collect clear and simple.
Furthermore, this document management system (FastReg) has been designed to allow you to create your own raw material database. That means that if several clients uses the same ingredients in their products, you don’t need to share the documents again, we already have what we need.
No more emails marked “URGENT!!!” by your clients asking you to share the COA of an ingredient that you have already shared at least 3 times with other brands. The process is more efficient and the number of email exchanges, drastically reduced!
If you are a contract manufacturer or a private labeler, you might be selling the same formula to several brands. In this case, why not offer a turnkey formula that would be:
By partnering with Taobé Consulting, you ensure your formula’s 100% compliance because it will be reviewed by certified Safety Assessors who are going to check each document individually, making sure all data match and that no present impurities are banned.
The registration process in Europe and the UK is always the same for all types of cosmetics, whether they are toothpaste, makeup, fragrances, SPF products or skincare creams. Each product needs a PIF, composed of a CPSR-A (unique to a formula) and a CPSR-B (unique to a unique product/brand).
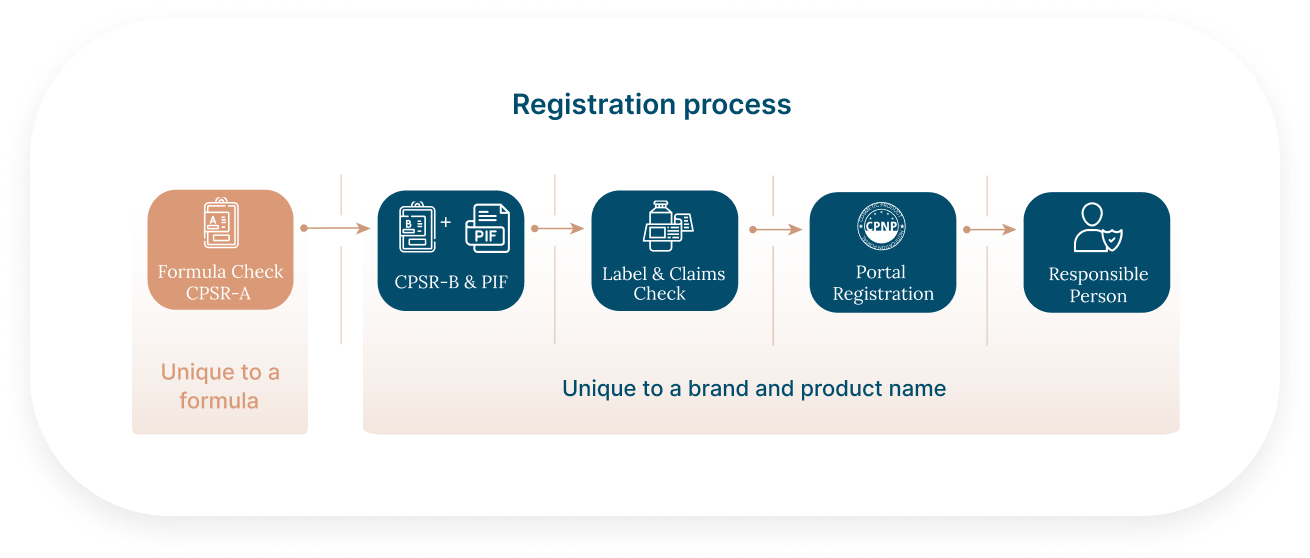
If you are selling the same formula to several cosmetic brands, then, it would add a considerable value to assess it for compliance in the EU and UK.
By taking care of the CPSR-A, you would:
Why don’t you give a try and start a first project with us?
You will see how easy we made it to move a product from development to compliance.
Octagon Point, 5 Cheapside
London EC2V 6AA, UK
47 Boulevard, Saint Michel
1040 Brussels, BE
C-615, 150, Dongtanyeongcheon-ro, Hwaseong-si, Gyeonggi-do, 18462
2025 © Taobe Consulting