Cosmetic product labels provide both mandatory information and voluntary marketing claims. This article breaks down the key elements of cosmetics regulation labels requirements, helping consumers understand the true meaning behind each item. By decrypting the content, we aim to offer clarity on what you’re buying and what you can expect in terms of safety, efficacy, and ethical considerations. Learn about the rules brands must follow and how trustworthy their labels really are.
Cosmetic Product Labelling: Mandatory Elements Under Article 19
Article 19 of the Cosmetics Regulation (EC) N° 1223/2009 outlines what must be included on cosmetic product labels. Divided into six paragraphs, the first specifies the essential information required before a product can be placed on the market.
Identifying the responsible person for cosmetic products in the EU
- Name or Registered Name and Address of the Responsible Person
Every cosmetic product sold in the EU must list a Responsible Person, typically the manufacturer, brand, or a delegated legal entity. This entity is accountable for the product’s safety and regulatory compliance. If an issue arises, EU authorities can contact the Responsible Person to access the Product Information File (PIF) for investigation.
The Responsible Person must be established within one of the EU 27 countries. Their name and address on the label confirm that a legal entity is accountable for the product, has submitted the necessary information via the Cosmetic Product Notification Portal (CPNP), and is subject to regulatory control. This ensures that safety and compliance assessments have been completed. Additionally, the Responsible Person must ensure the label adheres to all the requirements outlined in Article 19 of the Cosmetics Regulation.
As mentioned, the Responsible Person can be the manufacturer, brand, importer/distributor, or a third-party entity like Taobé, specialising in cosmetic product safety and compliance. Whoever assumes this role must rigorously assess the product before accepting responsibility. The stakes are high, as noncompliance can lead to heavy fines and even penal sanctions, including imprisonment.
Country of origin information
- The country of origin must be specified for imported cosmetics
This is where the familiar “Made in…” label comes in. Before Brexit, products manufactured and sold within the EU (including the UK) didn’t require a country of origin label. Now, with Brexit, EU brands selling in the UK and UK brands selling in the EU must indicate the country of origin.
According to the Rule of Origin, this is not determined by the shipping location but by where the product was produced or manufactured, giving the product its ‘economic nationality.’
Determining the country of origin is the final step in the customs clearance process. The earlier steps involve classifying the goods and establishing their value. Therefore, a product must demonstrate its origin before it can clear customs.
Nominal content requirements
- the nominal content at the time of packaging, given by weight or by volume, except in the case of packaging containing less than five grams or five millilitres, free samples and single-application packs
You will often see the net weight displayed in grams/millilitres (g/mL) and ounces/fluid ounces (oz/fl. oz.). This typically indicates that the brand sells its products both in Europe and internationally (e.g., in the US and Canada).
In the U.S., the general rule states that the measured content can be equal to or greater than the displayed net weight but never less.
In Europe, if you see a small ℮ symbol next to the weight, known as the Estimated symbol, it signifies that out of 100 units of cosmetics produced, a small percentage may vary slightly from the average weight displayed.
Since U.S. and EU regulations do not rely on the same calculations and results, many brands opt not to use the ℮ symbol, as it can complicate sales overseas. Additionally, its use requires specific audits and calibration of machinery conducted by an official accredited organisation.
The Responsible Person must review the technical documentation of the manufacturing process to ensure that this aspect is controlled and monitored by the producer. For each batch of product, such information must be included in the documentation submitted for the Product Information File creation.
Storage instructions and use by dates

- The date until which the cosmetic product, stored under appropriate conditions, will continue to fulfil its initial function and remain in conformity with Article 3 the “date of minimum durability.”
There are several checkpoints for the Responsible Person to review regarding this simple concept of storage conditions:
- Stored under appropriate conditions: Products intended for use in the bathroom, such as shower gel, shampoos, and soaps, are more susceptible to contamination by microorganisms than products stored in a drawer. The same applies to a sunscreen bottle taken to the beach.
- As a result, you may find storage instructions on your cosmetic creams, deodorants, sunscreens, and other products, such as: “store in a cool, dry place; do not place in direct sunlight; avoid heat.” These storage instructions are crucial for ensuring that the product remains effective and retains its properties throughout its lifespan.
- will remain in conformity with Article 3
Article 3 is about Safety. Accordingly, the expert who reviews the product needs to confirm:
- if the product is eligible for a Period After Opening or a Date of Minimum Durability
- the date until which the product remains safe after being opened.
The Period After Opening or PAO is represented by an open jar with a number of months placed next to it or within the jar symbol.
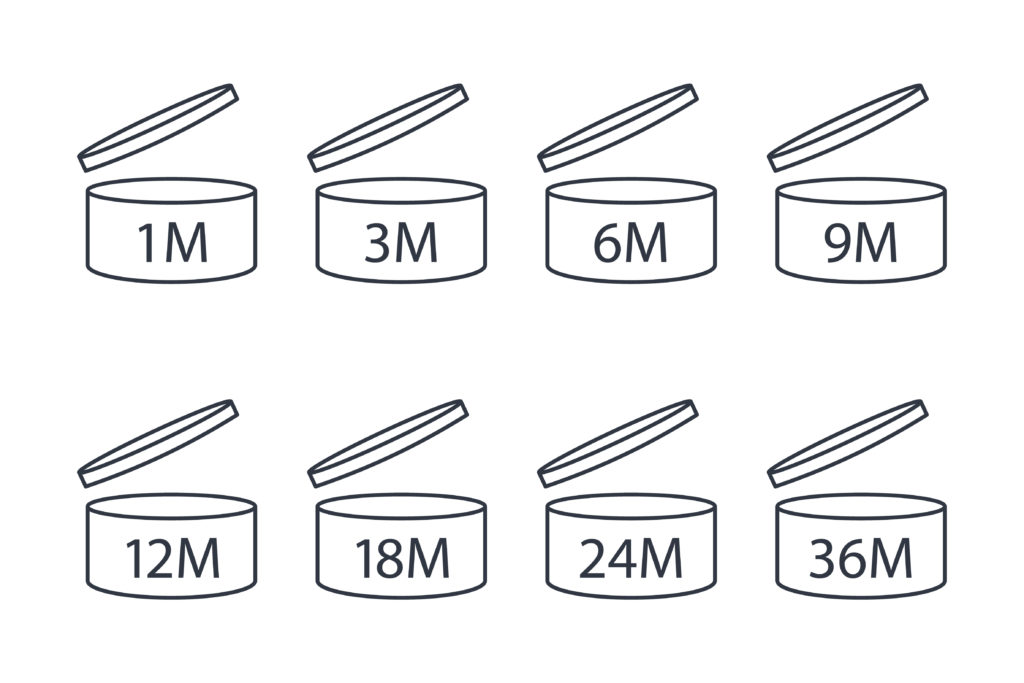
This symbol means that the product shelf life (meaning the time during which the product remains on a shelf unopened, in a store for instance) is equivalent or superior to 30 months. Once you buy and start using the product you may use it safely for 12 months.
The Date of Minimum Durability or DMD is represented by an hourglass next to which a date is written.
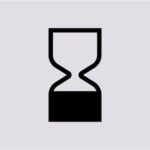
10/08/2025
This means that the product’s shelf life is inferior to 30 months and once you start using it, it remains safe until the date indicated next to the symbol.
Here, the distributor or shop where you can buy the cosmetic product plays an important role since he/she must ensure that the shelf life of the products they are selling has not passed.
There are a few products for which the concept of durability after opening is not relevant. This is the case for single use products which you use all at once. Or fragrances which contain a lot of alcohol and thus do not risk spoiling.
The Safety Assessor reviewing the product will consider the stability, microbiological quality, and preservation of the formula, along with the packaging used. For example, a pressurised container with no direct contact with air is less likely to spoil than a cream in a jar that you open and have direct contact with.
The category of the targeted consumers (adults vs. infants vs. elderly), the zone of application (eye vs. hair vs. body), and the quantity of product used per opening are also important factors. Therefore, similar to food products, it’s crucial to consider this information to ensure safety when using cosmetics.
How safety assessors determine label precautions
- Particular precautions must be observed in use, including those listed in Annexes III to VI, as well as any special precautionary information on cosmetic products for professional use.
When using a cosmetic product, it is generally intended for a specific purpose; for example, you would not use shampoo as shower gel, mascara as lipstick, or makeup remover wipes as diaper wipes. However, it’s also essential not to use body cream as an eye cream, for instance.
Why? Because the skin around the eyes is thinner than the skin on the legs or arms. Additionally, ingredients that may be suitable for body creams might not be appropriate for the eye area.
The Safety Assessor plays a key role in determining the precautions displayed on a cosmetic product’s label. By reviewing the formula, the expert calculates the risk of each ingredient (factoring in hazard, exposure, and dose) and confirms the conditions under which the risk is negligible. This assessment isn’t solely the Safety Assessor’s responsibility.
For ingredients with specific functions—such as preservatives, UV filters, colourants, or those subject to restrictions—the Scientific Committee for Consumer Safety (SCCS) defines safe concentrations and usage instructions. These precautions are vital for consumer safety and are displayed on the product label.
Reading and understanding these precautions before purchasing and using a cosmetic product is essential to ensure you know how to use the product correctly and safely.
The SCCS regularly reviews the safety of ingredients that could pose potential risks. Preservatives, often criticised by consumer associations as harmful chemicals, are essential for ensuring that cosmetic products remain safe during use. Without an effective preservative system, fungi, yeast, and mould can grow in the product.
This issue was recently seen in a sunscreen that used a “natural” preservative. Unfortunately, the chosen preservative wasn’t strong enough to protect against all microorganisms.
As mentioned in our Guide to the SCCS, this Committee determines the safe levels for preservative ingredients. Dismissing these ingredients could result in unsafe cosmetics. Even nature uses “toxic” methods to defend against parasites. With the rigorous checks performed before a cosmetic product reaches the market, you can trust those verified by a certified Safety Assessor.
Batch number as the product’s identification and traceability tool

- the batch number of manufacture or the reference for identifying the cosmetic product
The batch number serves as the product’s identification card. During the production of each batch, several verifications are carried out in compliance with GMP (Good Manufacturing Practices).
The specific standard for cosmetics, ISO 22716, must be followed by all manufacturers to ensure the product meets Cosmetics Regulation requirements. If a manufacturer does not follow this standard, they must prove that their manufacturing process covers all necessary checkpoints.
A batch refers to a set of products produced at a particular time for a specific market. It consists of a series of numbers and letters indicating the date, time of manufacture, the production line used, and the quantity produced at that time.
This system allows for tracking each cosmetic product throughout its lifecycle, from production to distribution. If an issue arises with a particular batch, the manufacturer or brand can easily recall or withdraw the affected products from the market.
As previously mentioned, ISO 22716 outlines the quality control measures required for each batch, ensuring that risks related to microbiological contamination, packaging deterioration, and other factors are mitigated. This framework is essential for maintaining consumer safety.
Understanding the function of cosmetic products: why it matters?
- the function of the cosmetic product, unless it is clear from its presentation
This may seem obvious, but it’s important to clearly understand what you are buying. For example, a tanning product should not be confused with sunscreen. With the expanding variety of cosmetic products and creative marketing designs and claims, it can sometimes be challenging to know exactly what you’re purchasing.
While items like mascara or lipstick have functions that are apparent from their appearance, other products, like a peeling foot mask, need to clearly state what they are and how to use them to avoid confusion with something like a hydrating face mask.
Additionally, it’s crucial to understand the product’s function in your language. With the globalisation of markets, English is often the default language used, but brands are required to translate essential information on their labels, including the product’s function, to ensure consumers know what they are buying.
If the Safety Assessor doesn’t fully understand the intended use of a product, they cannot evaluate its safety. The SCCS has established categories for cosmetic products that consider the targeted population, area of application, and other factors that impact the assessor’s calculations.
If a product cannot be classified into one of these categories, it cannot be placed on the market, as its notification to the regulatory portal requires clear classification.
Understanding the ingredient list on cosmetic products
- a list of ingredients
Most cosmetic ingredients must be named using the International Nomenclature of Cosmetic Ingredients (INCI). This system is regulated by the Personal Care Products Council (PCPC), a US-based organisation of scientific experts.
They are the only global authority responsible for assigning unique names to ingredients used in cosmetics, ensuring that all ingredients have a consistent name across all brands, at least in Latin characters.
The ingredient list must follow strict rules:
- Ingredients present at 1% or higher must be listed in descending order. This is why water (or Aqua in INCI) is often the first ingredient.
- Other ingredients, under 1%, can be listed in any order to prevent easy duplication of formulations.
- Fragrances and aromas must be labelled as PARFUM and AROMA.
- Colourants are listed at the end, by their Color Index (CI) number, and can be grouped when used in varying concentrations across a set of makeup products.
- For botanicals, the Latin names from the Linné system are used. For example, instead of Peppermint extract, you will see MENTHA PIPERITA EXTRACT.
- Allergens found in fragrances or aromas must be listed when present above specific thresholds for both leave-on and rinse-off products.
The cosmetic expert provides the manufacturer or brand with the correct list of ingredients, based on either their maximum or average concentrations in the product. A cosmetic product cannot list ingredients it doesn’t contain. Conversely, the list must include all the ingredients present in the product.
There’s no room for cheating, as authorities can easily detect whether an ingredient is missing or falsely listed, and violations can lead to serious repercussions.
Now that we’ve covered all the mandatory information, what else must appear on the packaging? In addition to the Cosmetics Regulation, other regulatory texts apply across consumer products.
These include:
- recycling symbols (determined at the national level)
- aerosol symbols (following the CLP Regulation)
- CLP elements (hazardous symbols like the Flammable symbol on products with a high alcohol content or in containers with gas)
Cosmetic product labelling: voluntary elements for brand marketing

Marketing is a brand’s superpower, making their products appealing and driving consumer purchases. But can brands say whatever they want? Claims—statements, logos, pictures—are marketing tools, but they are regulated, meaning brands cannot make unsupported claims. What they say must be proven.
In addition to the Cosmetics Regulation (EC) N° 1223/2009, COMMISSION REGULATION (EU) No 655/2013 of 10 July 2013 sets common criteria for justifying claims made about cosmetic products.
Some of these key principles include:
- Legal compliance
- Truthfulness
- Evidential support
- Honesty
- Fairness
- Informed decision-making
Here are some claim examples for each criterion.
- Legal compliance:
“Not tested on Animals” ???? Not allowed
The Cosmetics Regulation has banned animal testing on cosmetic products since 2004 and on cosmetic ingredients since 2009. This means that all products sold in Europe and the UK must comply with this ban. Claiming that a product respects this regulation is unnecessary because it simply adheres to the basic legal requirements.
2. Truthfulness:
“Contains Vitamin E” ⯑ Allowed if
This claim is allowed only if the ingredient is indeed present in the product and if its content is sufficiently high to be detected.
“Hydrating Cream” ???? Allowed
If the product contains a sufficient percentage of ingredients that are known to hydrate the superior layers of the skin, then the claim can be used on the cosmetic product.
3. Evidential support:
“Antioxidant properties” ⯑ Allowed if
The product can claim it has antioxidant properties if it can be demonstrated that its formula contains an effective concentration of ingredients that have antioxidant properties.
4. Honesty:
“Will make your skin look like baby skin” ???? Not allowed
No cosmetic product can claim that it can revert the signs of ageing and make you appear young again. This would go beyond the available supporting evidence.
5. Fairness:
“Paraben free” ???? Not allowed
Parabens, even if under scrutiny for their safety, are allowed to be used in cosmetic products. A brand cannot claim it does not contain parabens because it would imply that the products that contain them are not safe.
6. Informed decision-making
“Fragrance free” ???? Allowed
Some consumers – because of allergies – prefer buying products that do not contain fragrance ingredients. Although fragrances are allowed ingredients, this claim allows sensitive consumers to make an informed choice.
What do authorities do when they inspect cosmetic products labels?
When authorities inspect cosmetic products labels, inspections typically happen at the point of sale, often at the distributor’s location. Inspectors first review the labels and may purchase a product for further testing. They check various aspects such as composition, claims, and potential impurities like heavy metals.
The compliance of the label and the claims provides important insights into the product’s conformity with regulations. If issues arise, such as a banned ingredient listed, authorities will question the Responsible Person, who must justify the label’s non-compliance.
Conclusion
Cosmetic products labels can be trusted as long as they have undergone thorough review by safety assessors and confirmed for compliance. The Responsible Person must ensure this prior to the products being placed on the market, as the notification of products on the Cosmetic Product Notification Portal (CPNP) must include the final labels.
Reputable Responsible Persons hire safety assessors to verify both safety and compliance. After ensuring that all mandatory items are present and accurately displayed, the expert will request sufficient evidence for each claim made on the label.
Numerous tools are available to help brands comply with the Cosmetics Regulation, the Common Criteria for claims, and other relevant regulations. Consumers also play a crucial role in maintaining the quality of cosmetic products on the market by choosing official and reputable distribution channels. Quality comes at a cost.
As demonstrated, brands must invest in testing, verification, review, and assessment to ensure compliance. With this information, we hope you now have the knowledge to choose your cosmetics wisely.
Author: Sophie Noiset, Quality Responsible and Operations Director
Disclaimer: this article in no way replaces a scientific article on the subject, but its aim is to popularise and educate everyone in the concepts of Cosmetic Regulations. If you need professional help, please feel free to contact us.



