If you’re in the cosmetics development or manufacturing sector and need expert advice on ingredient safety, Taobé Consulting is here to help. We provide thorough and scientifically supported cosmetic ingredient reviews, ensuring compliance and safety.
Our team brings a wealth of knowledge and experience to support you in understanding the safety aspects of cosmetic ingredients. Obtaining a toxicological evaluation of your cosmetic ingredients may help you access international markets and big stakeholders of the cosmetics industry.
Below, we provide all the necessary information about what is included in our cosmetic ingredient review services, detailing the essential components and processes that ensure thorough assessments.
Review of the safety of cosmetic raw material and/or ingredient… and more
Taobé can evaluate the toxicology of your cosmetic ingredients and then can help you with the registration of your products according to your specific market.
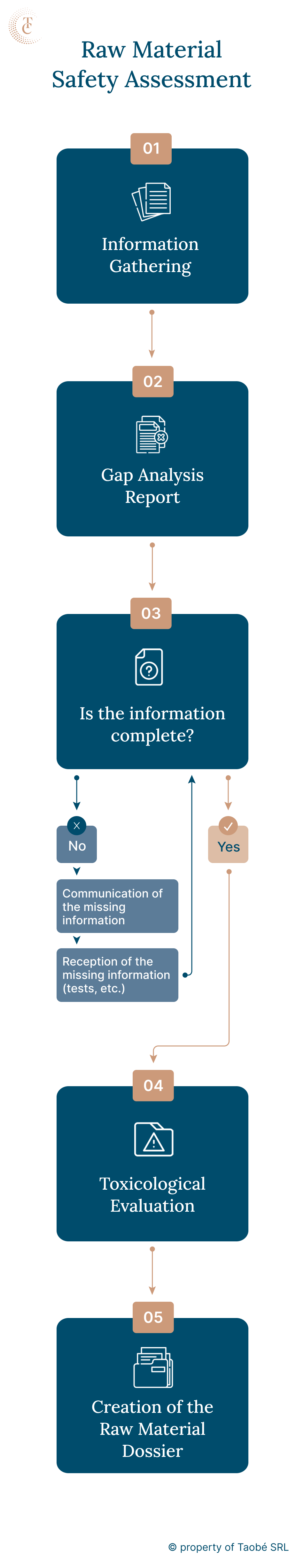
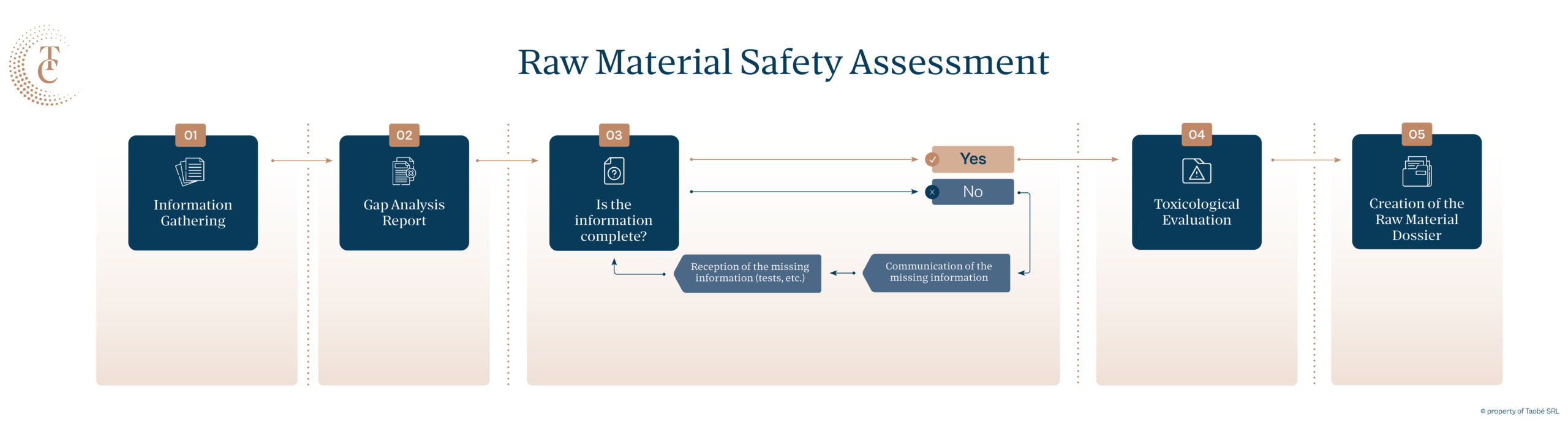
Safety assessments are essential not only for finished cosmetic products but also for the raw materials and ingredients used in their production. Raw materials intended for the European market must meet specific requirements to ensure their safety, quality, and compliance.
A comprehensive toxicological evaluation of cosmetic raw materials is crucial for manufacturers and suppliers aiming to sell their products to major clients in Europe. This means that the cosmetic raw materials must have:
Cosmetic raw materials are composed of one or more cosmetic ingredients, which are chemical substances.
A toxicological evaluation at Taobé is conducted by a qualified safety assessor. This process involves calculating, compiling, and researching toxicological data on specific substances where such information has not previously been compiled or is not readily available in open sources.
To assess a chemical substance, Taobé’s safety assessors utilise the Notes of Guidance for Testing and Safety Evaluation (12th Revision) issued by the Scientific Committee on Consumer Safety (SCCS). This document provides a comprehensive framework for our evaluations, ensuring thoroughness and compliance with current standards.
The toxicological evaluation report from Taobé Consulting will contain:
The duration of the toxicological evaluation can vary based on several factors:
For a comprehensive guide on the process involved in evaluating and reporting on cosmetic raw materials, refer to our detailed article on toxicological evaluations of cosmetic raw materials.
A cosmetic ingredient review evaluates the safety, toxicology, and regulatory status of raw materials or ingredients to confirm they can be safely used in cosmetic formulas.
Toxicological evaluations help brands demonstrate ingredient safety, meet regulatory obligations, and gain access to major retailers, manufacturers, and international markets.
Assessments include toxicological profiles, ingredient safety data, regulatory status, quality information, physical-chemical characteristics, and recommended maximum safe use levels.
Yes. Each ingredient must have toxicology information to support safety of the raw material as a whole.
You receive a full toxicological assessment report, a CLP Safety Data Sheet, and a Cosmetic Raw Material Regulatory Identity Card with regulatory details and safe-use concentrations.
Yes. The assessment verifies whether raw materials meet EU requirements, including restrictions, purity criteria, and safety expectations for cosmetic use.
Timelines vary depending on the complexity of the raw material, availability of toxicology data, and whether additional tests are required.
Taobé identifies gaps and advises on the additional testing or documentation needed to complete the evaluation.
Yes. Ingredient safety is required to complete CPSRs for finished products, making raw material assessments essential for compliant product development.
Yes. Comprehensive toxicological data supports regulatory submissions, enabling access to Europe, the UK, and other regulated markets.

Would you like Taobé to review the safety of your cosmetic raw material and/or ingredient?
Please do not hesitate to contact us if you have any questions about the toxicological evaluation of your cosmetic ingredients.