Manufacturing cosmetic products involves numerous quality decisions and responsibilities, including raw material selection, formula compliance, meeting clients’ expectations, adhering to market requirements, and data collection.
What if you had a regulatory partner to relieve you from some of this burden, making your life and the lives of the cosmetic brands you work with much easier?
We can:
At Taobé, we work directly with cosmetic raw material suppliers on INCI registration, (eco-) toxicological profiles, safety assessments, claims efficacy, and more. This allows us to build a comprehensive database of ingredients with specific characteristics (vegan, natural, innovative, etc.), all fully documented according to Cosmetics Regulation requirements.
Share with us the specifications of the cosmetic raw materials you are looking for (e.g., SPF, natural, vegan, Japan), and we will prepare a shortlist of ingredients from our database that match your criteria. If we do not have exactly what you need, we will connect you directly with the raw material suppliers we collaborate with.
When we work successfully with cosmetic manufacturers who consistently provide high-quality and thorough documentation in a timely manner, we list them in our “best choice” database, which we promote among our international clientele.
We can also direct clients seeking a contract manufacturer or private labeller to your sales department, depending on the product category and the labelling claims they wish to make.
So, let’s become trusted partners!
To register a product in Europe and the UK, a Product Information File must be created, containing all documents and information related to the product and its ingredients. This means that all confidential information about a product—such as the formula, raw material trade names, and suppliers—would need to be disclosed to review the formula compliance.
Without proper safeguards, a contract manufacturer would have to disclose this valuable know-how to the cosmetic brand in order for the brand to expand in Europe and other markets.
By partnering with Taobé Consulting, you create a secure buffer between your business and the client. No confidential data will ever be shared with the brand, as a NDA will be signed, and Taobé will manage all registration steps while keeping all product data secure. This way, all parties achieve their goals: the brand can expand internationally, and the manufacturer maintains complete confidentiality.
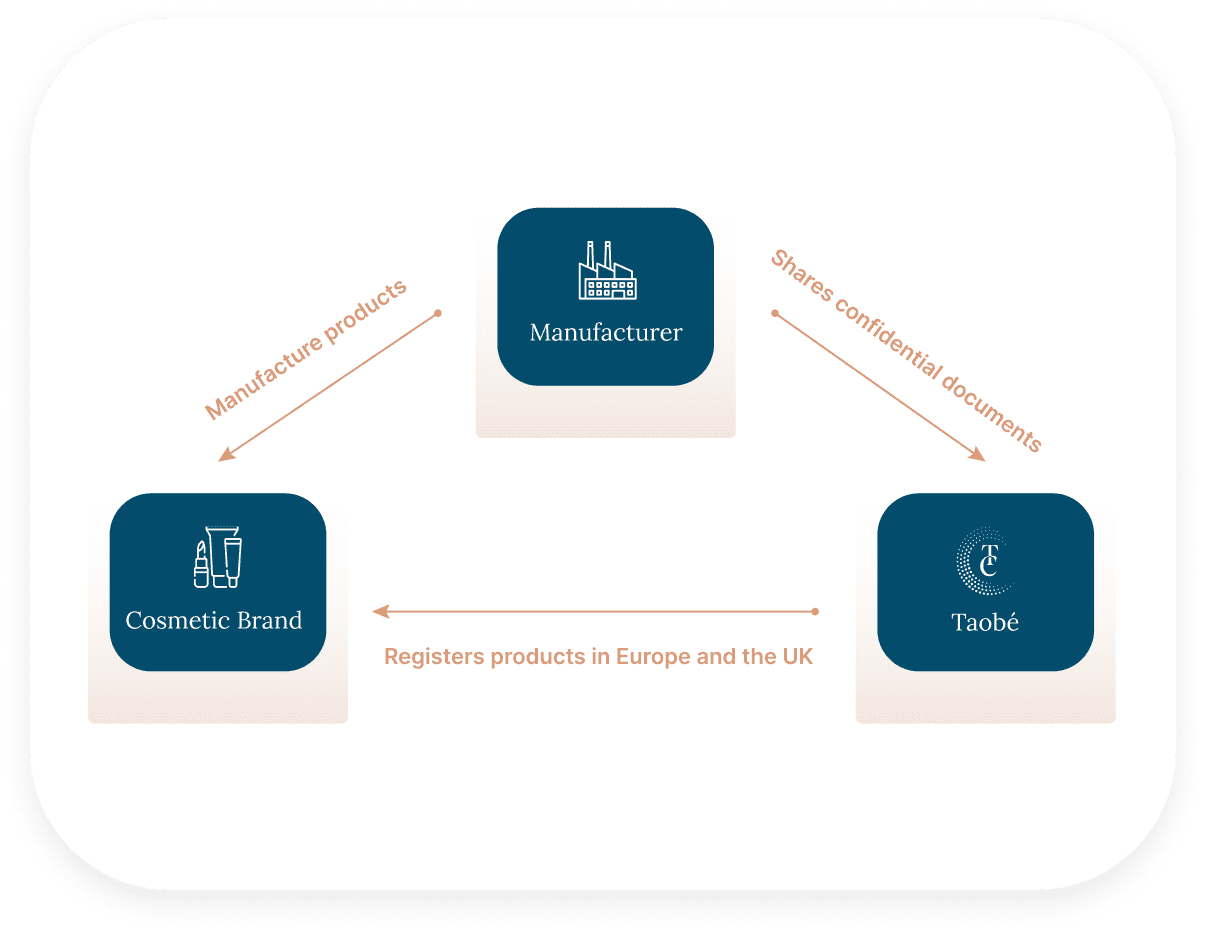
Working with multiple cosmetic brands seeking to expand in Europe, the UK and the US can be time-consuming, as the same confidential documents often need to be shared several times with different contacts.
By partnering with Taobé Consulting, you only share your documents once, with a single point of contact, through our secure document management system, FastReg. This system keeps the document collection clear and simple, and also allows you to create your own raw materials database. If multiple clients use the same ingredients, you don’t need to share the documents again—we already have everything we need.
No more urgent emails from clients requesting COAs or other documentation you’ve already shared with other brands. The process is more efficient, reducing the number of email exchanges and simplifying your workflow.
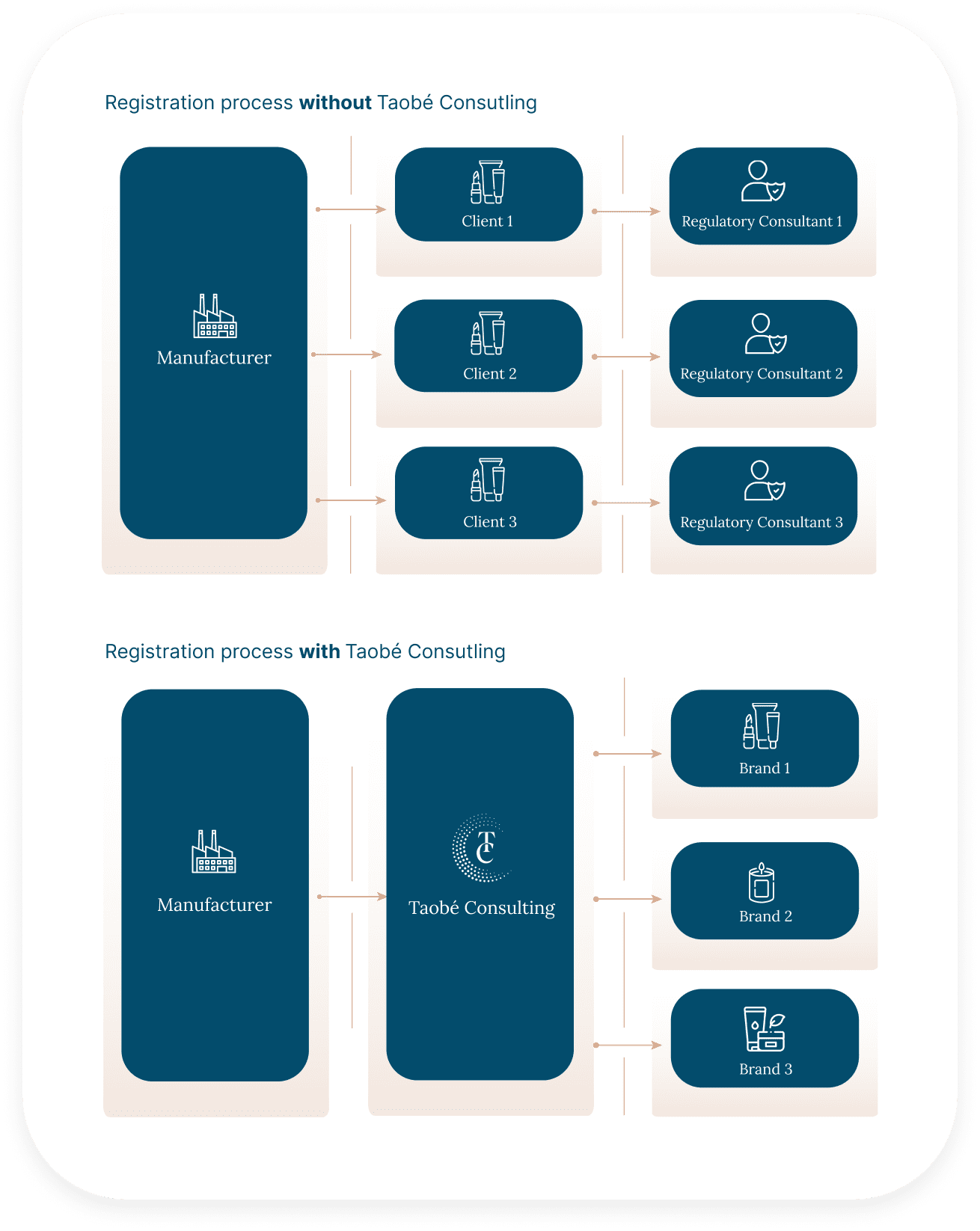
If you are a contract manufacturer or private labeller selling the same formula to multiple brands, you can offer a turnkey solution that is:
By partnering with Taobé Consulting, you ensure your formula is fully compliant. Certified Safety Assessors review each document individually, verifying that all data is accurate and that no prohibited impurities are present. And you get an audit-proof formula compliance report.
The registration process in Europe and the UK is consistent across all cosmetic types, including toothpaste, makeup, fragrances, SPF products, and skincare creams. Each product requires a Product Information File (PIF), which includes a CPSR-A (unique to the formula) and a CPSR-B (unique to the product/brand).
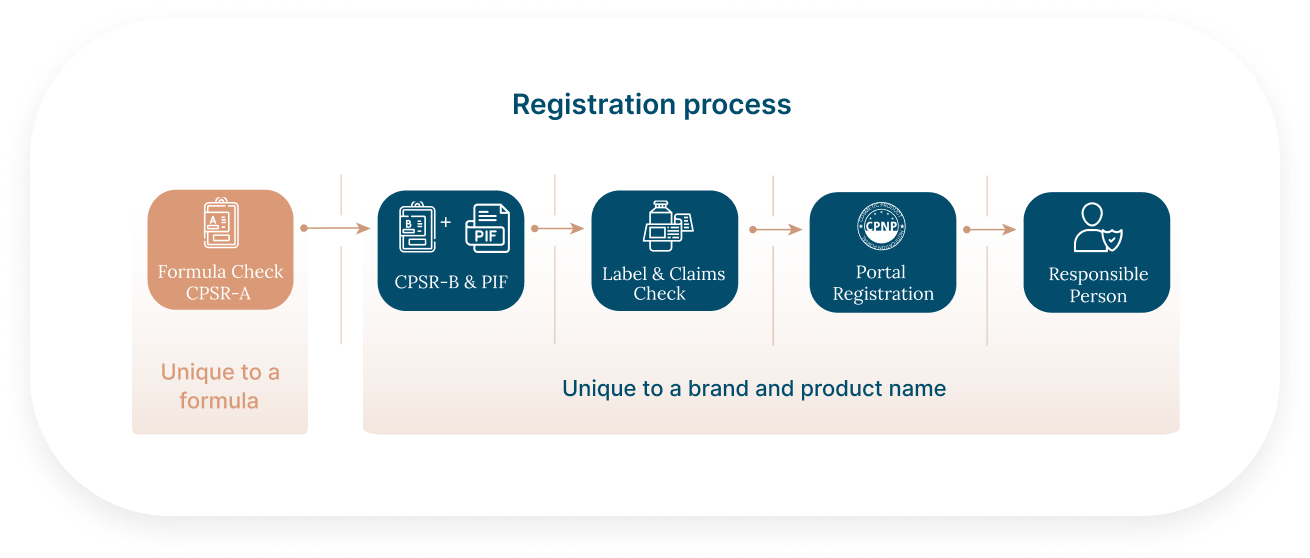
If you are selling the same formula to several cosmetic brands, assessing formula compliance in the EU, UK and US can add considerable value.
By having the CPSR-A prepared and reviewed, you would:
It’s when a manufacturer produces cosmetic products for a brand using their own formulas or private-label formulas.
They manage compliance, safety assessments, documentation, and registrations needed to place cosmetic products on global markets.
Yes, if the formula is unchanged and has a compliant CPSR-A, it can be used for several cosmetic products across different brands.
Confidential formula data, raw material lists, and supplier details remain protected under NDAs handled only by the regulatory partner.
Yes. Ingredient sourcing and access to trusted cosmetic raw material suppliers can be part of the support.
A pre-assessed formula speeds up market entry, confirms safety, and increases its value for brands purchasing private-label cosmetic products.
A centralised system stores SDS, CoA, and raw material documents once, reducing repeated requests from different cosmetic brands.
Yes. The CPSR-A confirms the safety and compliance of every cosmetic product formula before it can be sold to multiple brands.
Yes. Multi-market compliance guidance ensures cosmetic products meet each region’s regulatory requirements.
They should look for strong documentation, reliable raw material sourcing, proven compliance processes, and confidentiality protection.

Start your first project with us and discover how simple we make the journey from product development to full compliance. With our support, you can move products to market faster, confidently, and without hassle.